Leeds University
Designing a Disposable Internal Scaffold for Laparoscopic Surgery
Impact: xxxxx
Brief
To transform a complex, costly surgical prototype into a manufacturable, disposable medical device that could support laparoscopic procedures while reducing costs and improving usability.
Services
Concept Development, Prototyping, Design for Manufacture, IP Support
Deliverables
Concept Development, Prototyping, Design for Manufacture, IP Support


“We commissioned Pd-m to convert a medical device concept into an innovative, realisable and manufacturable product for licensing. The project required Richard and his team to work closely with a multidisciplinary team including our Surgical Technologies research group, surgeons at Leeds Teaching Hospitals and the commercial partners. Pd-m helped us achieve the desired outcome, they are a professional innovation consultancy and I can highly recommend them.”
Case Study
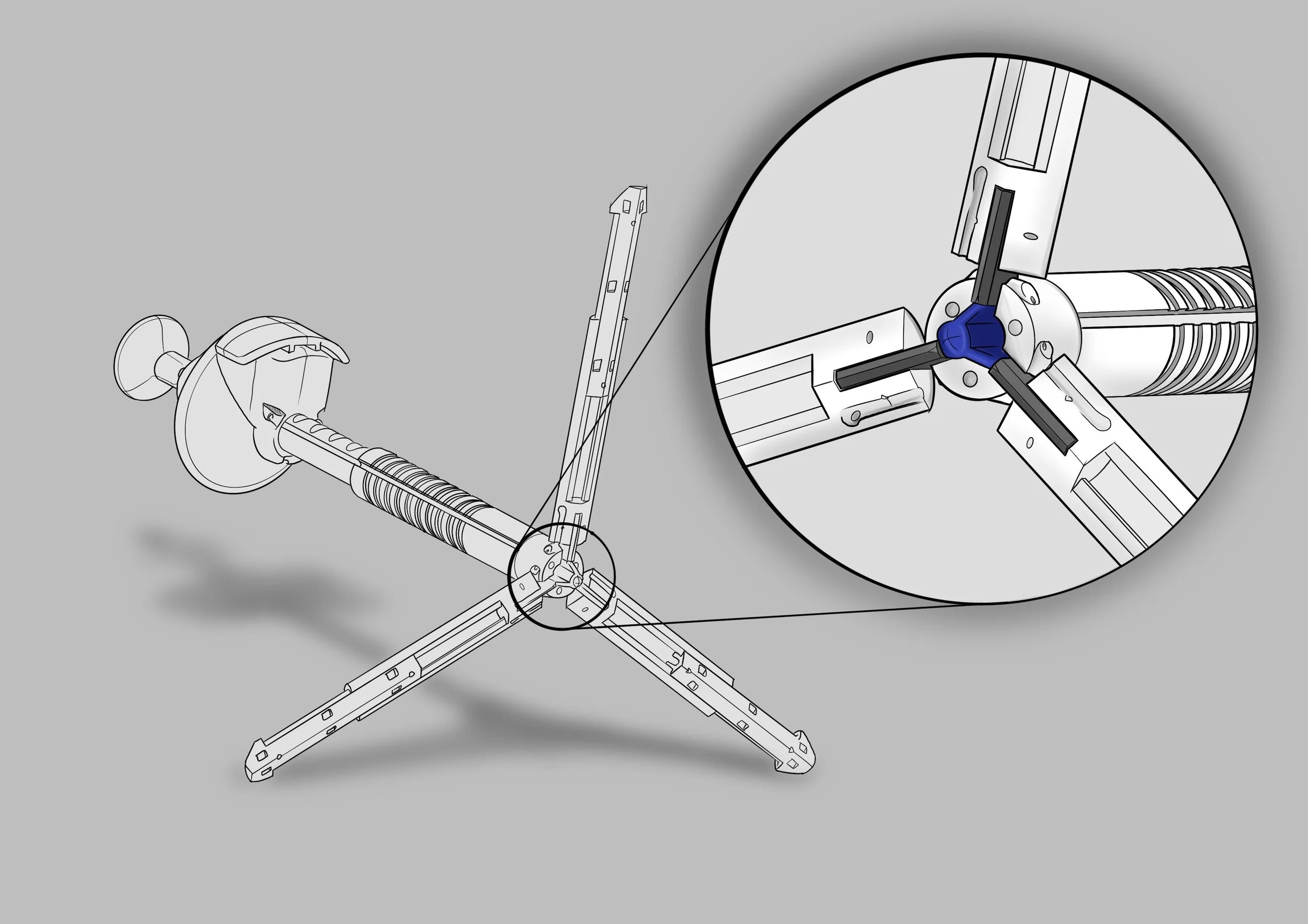
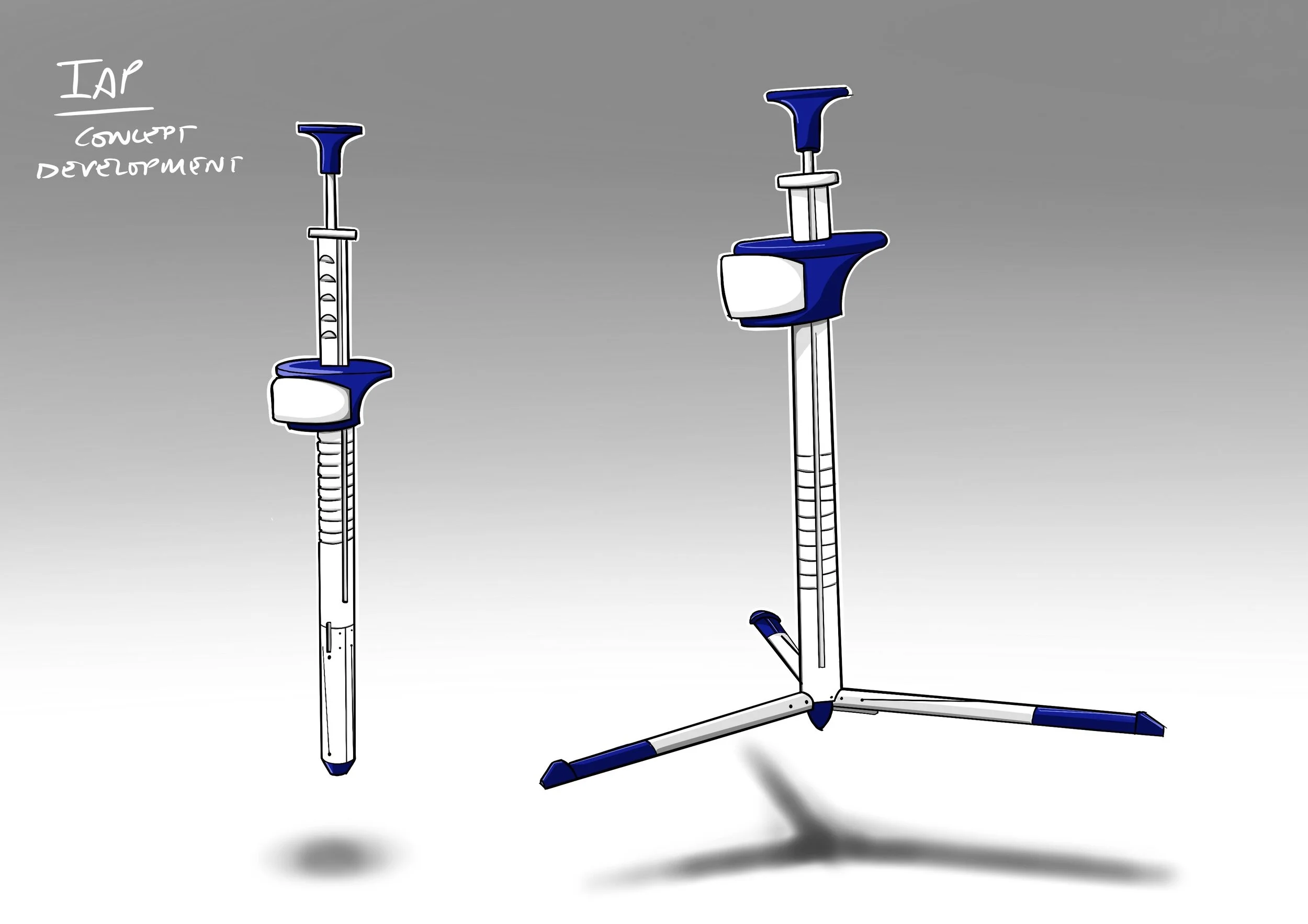
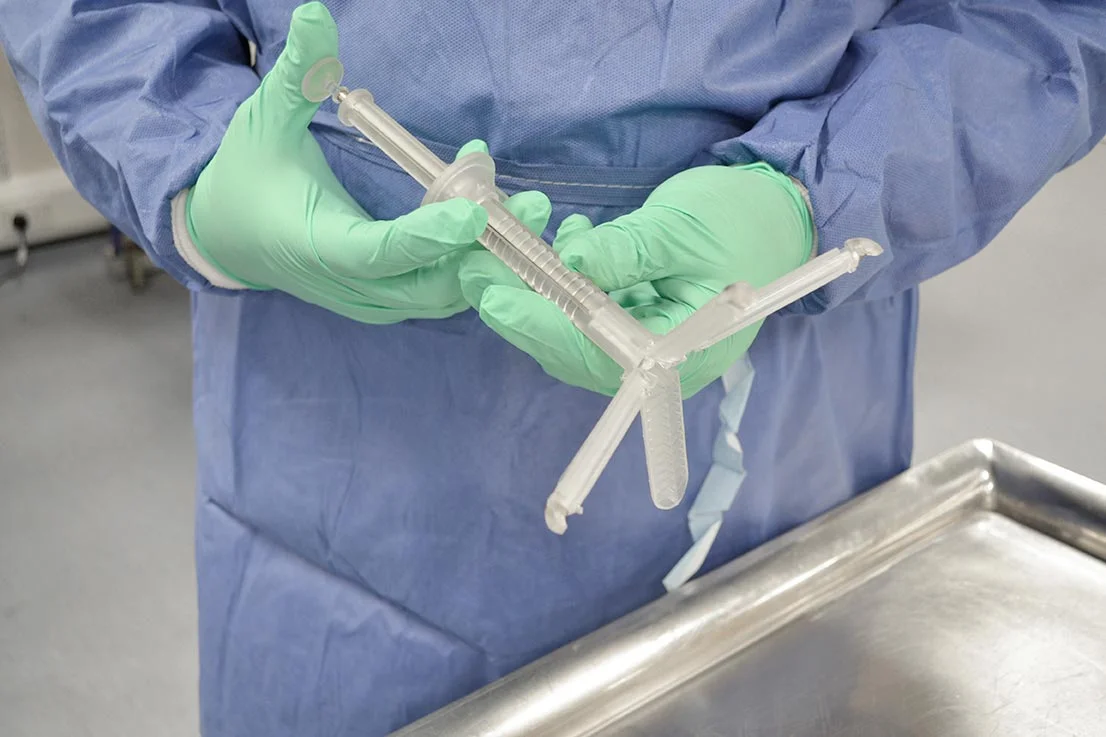
The Intra-Abdominal Retraction Platform (IARP) is an internal scaffold structure, deployed through a port, to aid procedures in laparoscopic surgery. The concept originated from a leading surgeon, who produced an initial prototype, but while it proved feasibility, it was prohibitively expensive and complex to manufacture.
To bring it closer to clinical adoption, the challenge was to simplify, reduce cost, and engineer a product fit for surgical teams and scalable manufacturing.
Our Approach
Over the course of eight design evolutions, we worked to refine every aspect of the scaffold structure. Each round balanced surgeon usability, functional performance, and manufacture efficiency. By rethinking the assembly and material choices, we dramatically reduced complexity without compromising on surgical utility.
Designing out cost
One of the key breakthroughs was driving down the cost of production—ultimately achieving a unit cost 100 times less than the original prototype. At the same time, we designed the product to be single-use disposable, which avoided the costly and time-intensive sterilisation process that reusable instruments require.
The Result
The final outcome was a patented product solution, licensed to a manufacturer and distributor. By involving both the Surgical Technologies research group and surgeons at Leeds Teaching Hospitals, the design journey was clinically informed at every stage, ensuring both practicality in theatre and commercial viability.
